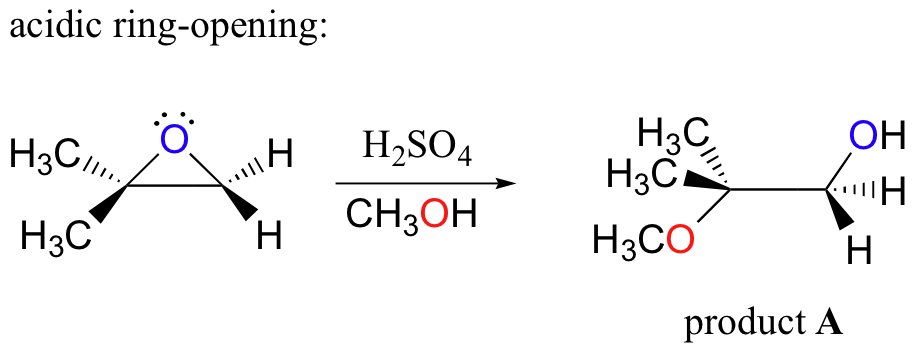
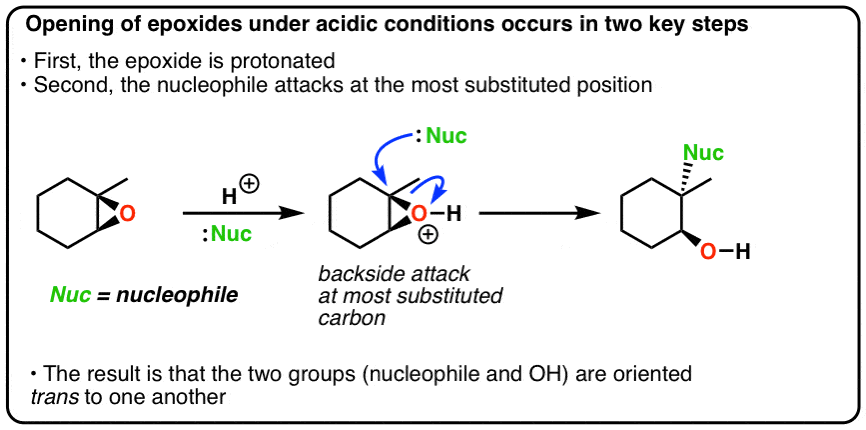
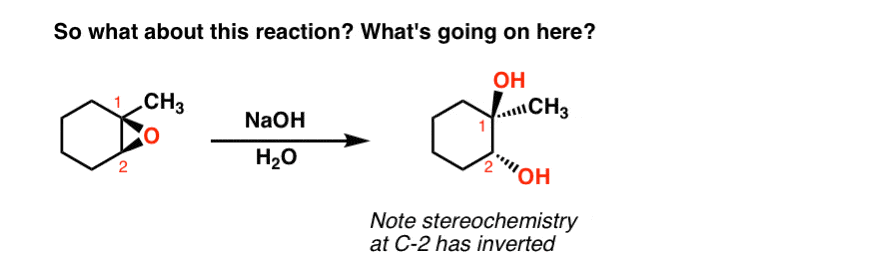
It is a hybrid of both. Although the acid-catalyzed ring-opening of epoxides follows a mechanism with S N 2 features inversion of stereochemistry no carbocation rearrangements the mechanism is not strictly a S N 2 mechanism.
9 14 Opening Of Epoxides Acidic Versus Basic Conditions Chemistry Libretexts
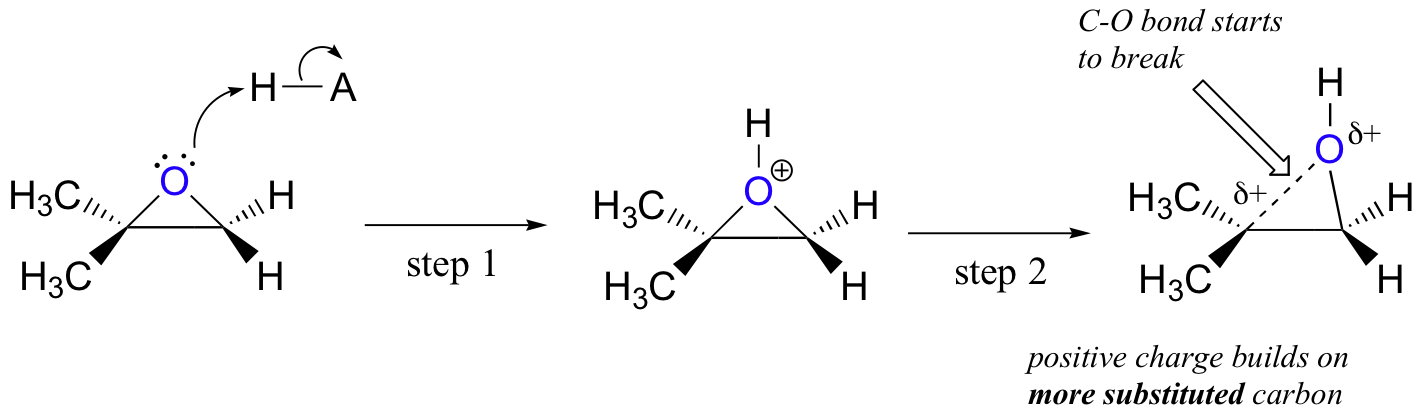
In the first step the oxygen gets protonated.

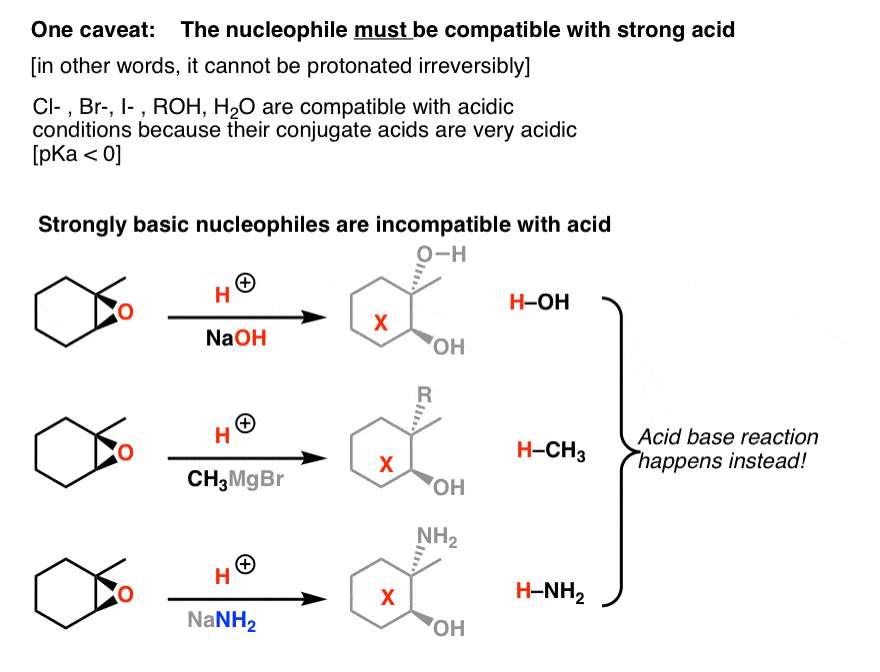
Acid catalyzed ring opening of epoxides. Electrostatic considerations have greater importance with a protonated intermediate. In conclusion the salenCo-catalyzed epoxide ring opening with carboxylic acids provides convenient access to synthetically useful products and the first indication of generality in nucleophile- epoxide reactions catalyzed by chiral salen complexes. Herein we wish to report the ring-opening reactions of MCPs and epoxides with aromatic amines and alcohols catalyzed by Lewis acids in the presence of perfluorinated compounds in scCO 2 which offer a way to produce various alcohols amino-alcohols homoallylic amines and ethers under an environmentally benign condition.
First the oxygen is protonated creating a good leaving group step 1 below. Vinyl alcohols yield tautomers. The mechanism of how halohydrins make epoxides via intramolecular SN2.
Markovnikov addition of alcohols yields ketones. In acid catalyzed ring-opening the epoxide oxygen is protonated under acidic conditions activating it for nucleophilic attack. Likewise polyols and nitrate esters are produced in solutions of HNO 3 NaNO 3.
Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. Polyols and sulfate esters are observed from the ring-opening of the epoxides in solutions of H 2 SO 4 Na 2 SO 4. Acid Catalyzed Epoxide Ring Opening.
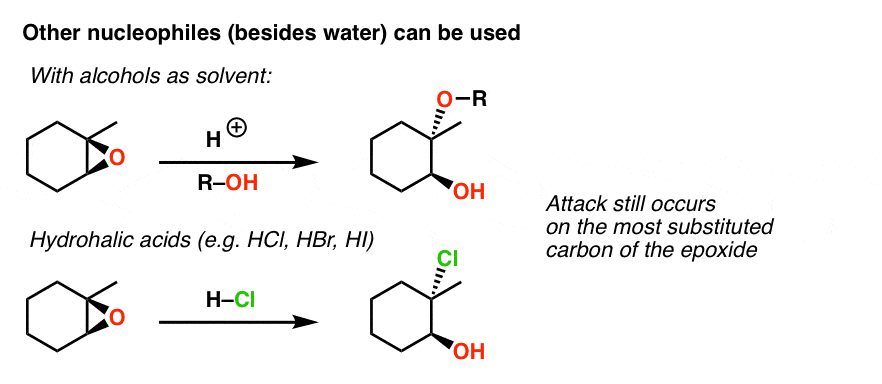
Double halogenation of alkynes. The ring opening reactions are commonly catalyzed using strong acids Lewis bases or Lewis acids. While strong acids are important industrially they tend to result in low regioselectivity for the epoxide ring opening with alcohols.
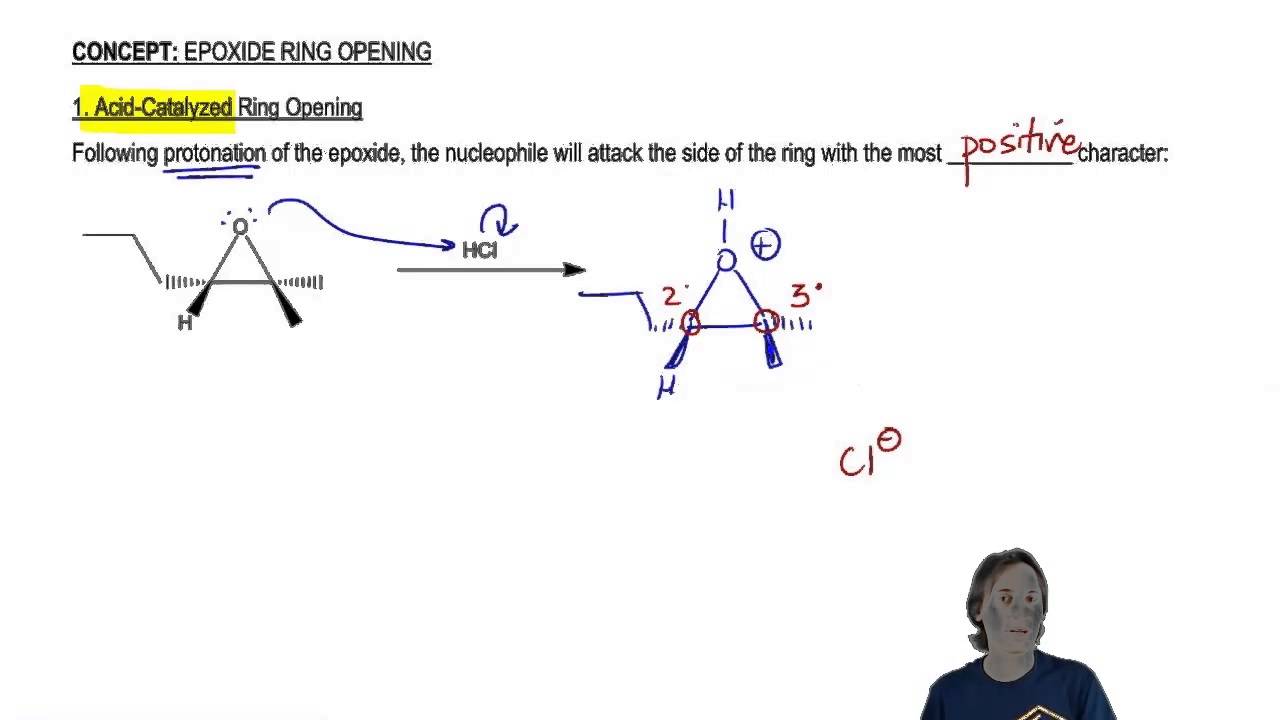
Nucleophile prefers 3 1 2. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. Epoxides experience a great deal of ring strain and as such are rather reactive.
Acid-Catalyzed Ring Opening of Epoxides. First the oxygen is protonated creating a good leaving group step 1 below. In sulfuric acid the rate of acid-catalyzed ring-opening is dependent on.
They undergo ring-opening reactions under acidic or basicnucleophilic conditions. Acid Catalyzed Ring Opening. In the case of acid catalysed ring opening the mechanism is not exactly SN1 or SN2.
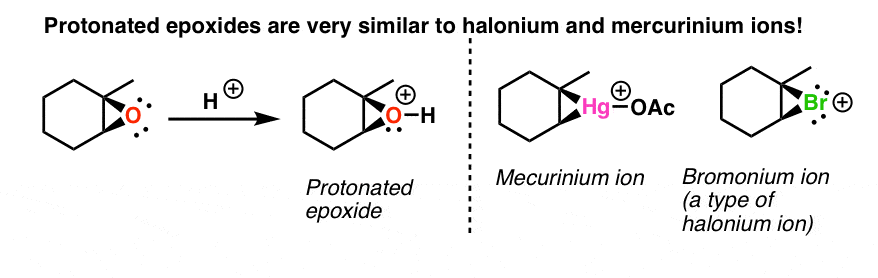
Epoxides also known as oxiranes are three-membered ring structures in which one of the vertices is an oxygen and the other two are carbons. The acid-catalyzed epoxide ring-opening reaction mechanism is analogous to the formation of the bromonium ion in halogenation of alkenes and mercurium ion formation in oxymercurationdemercuratioin or alkoxymercurationdemercuration. First the oxygen is protonated creating a good leaving group step 1 below.
The transitition state has more progress toward the C-LG bond breaking than an S N 2 but more progress toward the C-Nu bond forming than S N 1. Double hydrohalogenation of alkynes. The carbons in an epoxide group are very reactive electrophiles due in large part to the fact that substantial ring strain is relieved when the ring opens upon nucleophilic attack.
General properties of double addition reactions to alkynes. When an asymmetric epoxide undergoes solvolysis in basic methanol ring-opening occurs by an SN2 mechanism and the less substituted carbon is the site of nucleophilic attack. A model system consisting of methyloxirane formate and formic acid was used to study the nucleophile-catalyzed and nucleophile and acid-catalyzed opening of an epoxide ring using ab initio.
Opening Of Epoxides With Acid Master Organic Chemistry
Opening Of Epoxides With Acid Master Organic Chemistry
Opening Of Epoxides With Acid Master Organic Chemistry

Pin On Aldehydes And Ketones Practice Problems
9 14 Opening Of Epoxides Acidic Versus Basic Conditions Chemistry Libretexts
Opening Of Epoxides With Acid Master Organic Chemistry

Acid Catalyzed Ring Opening Of Epoxide Chemistry Stack Exchange
Opening Of Epoxides With Acid Master Organic Chemistry
Acid Catalyzed Epoxide Ring Opening Youtube
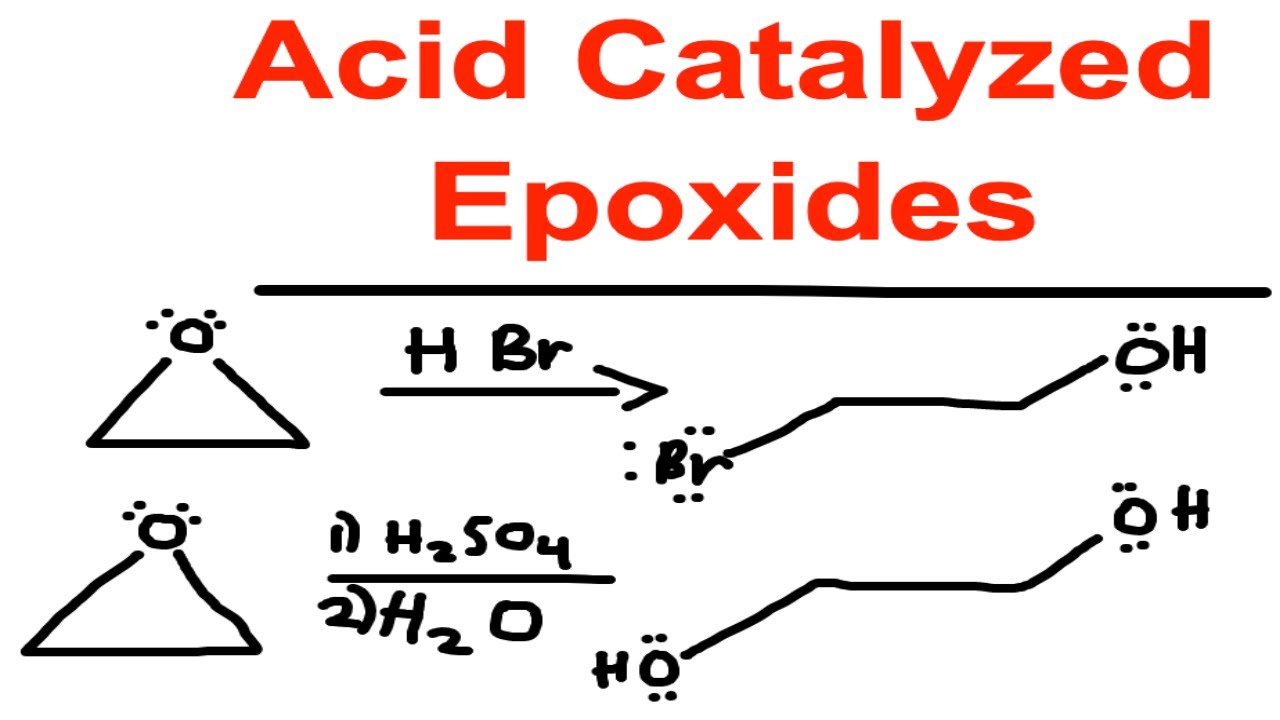
Acid Catalyze Ring Opening Of Epoxides Hbr Hcl Hi H2so4 H20 Organic Chemistry Youtube

Pin On Aldehydes And Ketones Practice Problems
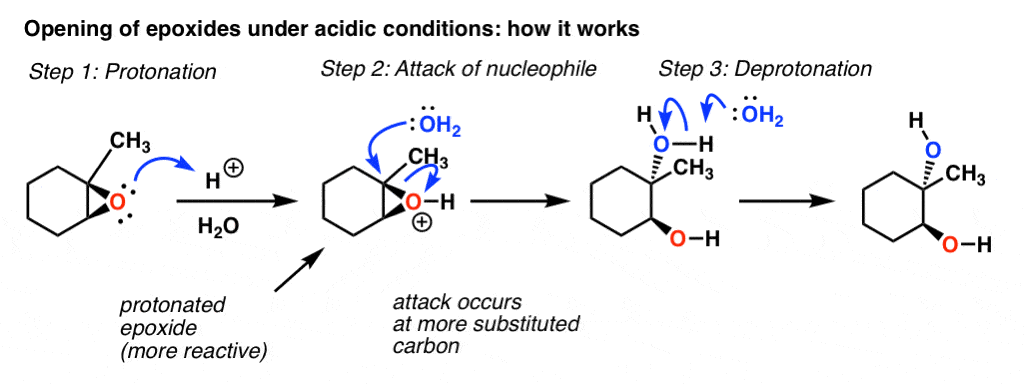
Opening Of Epoxides With Acid Master Organic Chemistry
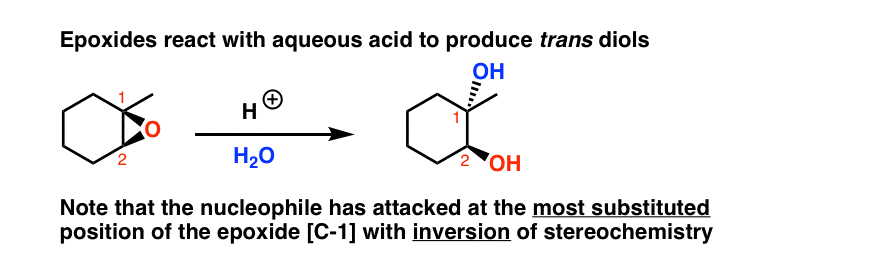
Opening Of Epoxides With Acid Master Organic Chemistry
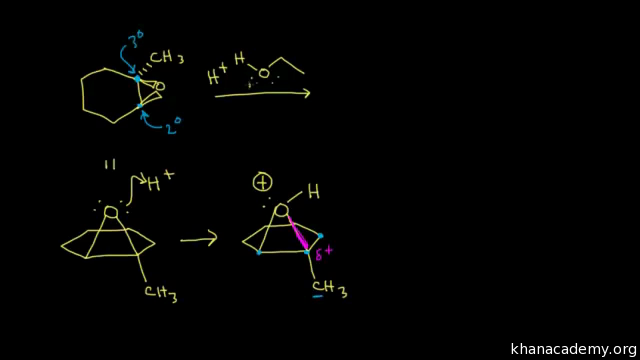
Ring Opening Reactions Of Epoxides Acid Catalyzed Video Khan Academy

Pin On Alkyne Reactions With Practice Problems

Pin On Reactions Of Alcohols With Practice Problems

Epoxide Reactions Acid Catalyzed Ring Opening Chem 2211 Docsity

Pin On Reactions Of Alcohols With Practice Problems

0 comments